Uranyless Aqueous
Uranyless Aqueous
Uranyless aqueous is a contrasting solution that substitutes for uranyl acetate. As recommended by Reynolds (1963), it is highly advisable to reinforce the initial contrast (in our case, UranyLess) with lead citrate in a NaOH-saturated atmosphere to prevent contamination from atmospheric CO2.
To facilitate the use of lead citrate during contrasting, it is packaged in AirLess bottles (without air and thus without CO2). You can store this 30 ml bottle for a long time without the risk of CO2 contamination, a common problem for microscopists.
The 30 ml AirLess bottle packaging allows you to contrast over 1500 grids.
This packaging is doubly effective: it provides long shelf life and generates minimal waste.
For using UranyLess aqueous in automated contrast devices like Leica’s EM Stain, UranyLess is available in a 200 ml bottle. It is worth noting that UranyLess aqueous is packaged in an AirLess bottle to facilitate drop dispensing only, as it is not affected by air or light.
Check out our results
Yeast
Preparation of the sample according to the following protocol:
- Standard fixation with Glutaraldehyde – Osmium – Embedding in Epon.
- Contrast with UranyLess followed by Lead Citrate.
Here are some results from Jeannine Lherminier (INRA – Dijon).
Photographs taken using Transmission Electron Microscopy
Trematodes
We present some results from Yann Quilichini (Microscopy Platform at the University of Corsica – Corte).
Sample preparation according to the following protocol:
- Standard fixation with glutaraldehyde, osmium, embedding in Spurr resin.
- Thin sections – contrast with aqueous UranyLess followed by lead citrate (according to Reynolds).
Photographs taken using Transmission Electron Microscopy.
Polymersomes (Polymere)
The IMRCP Laboratory in Toulouse, led by Anne-Françoise Mingotaud, tested UranyLess in comparison to uranyl acetate, which is at an acidic pH of 4 (seems to disrupt the molecular structure organization). They also compared observations using Cryo-SEM (scanning electron microscopy).
Observation using Scanning Electron Microscopy in Cryo mode and by negative staining with UranyLess.
Reconstructed Epidermis (human)
We present the results from Audrey Houcine (CMEAB Toulouse).
Sample preparation according to the following protocol:
- Standard fixation with glutaraldehyde, osmium, Epon/Araldite
- Ultrathin sectioning, double contrast with Uranyless followed by lead citrate
Photographs taken using Hitachi HT7700 Transmission Electron Microscopy by Audrey Houcine.
Muscle-Nerve (mouse)
Sample preparation according to the following protocol:
- Standard fixation with glutaraldehyde, osmium, Epon
- Ultrathin sectioning, double contrast with Uranyless 1min followed by lead citrate 1min
Ovarian Follicle (mouse).
Sample preparation according to the following protocol:
- Standard fixation with glutaraldehyde Fixative PFA, osmium, Epon
- Ultrathin sectioning, double contrast with Uranyless 1min followed by lead citrate 1min
Kidney (mouse)
Mouse Kidney
Sample preparation according to the following protocol:
- Classic PFA- Glutaraldehyde, Osmium, Epon fixing.
- Ultrafine section – 1 minute UranyLess contrast – 1 minute Lead Citrate.
Photos taken with a Hitachi HT7700 Transmission Electron Microscope by Nacer BENMERADI (R&D – Delta Microscopies – France).
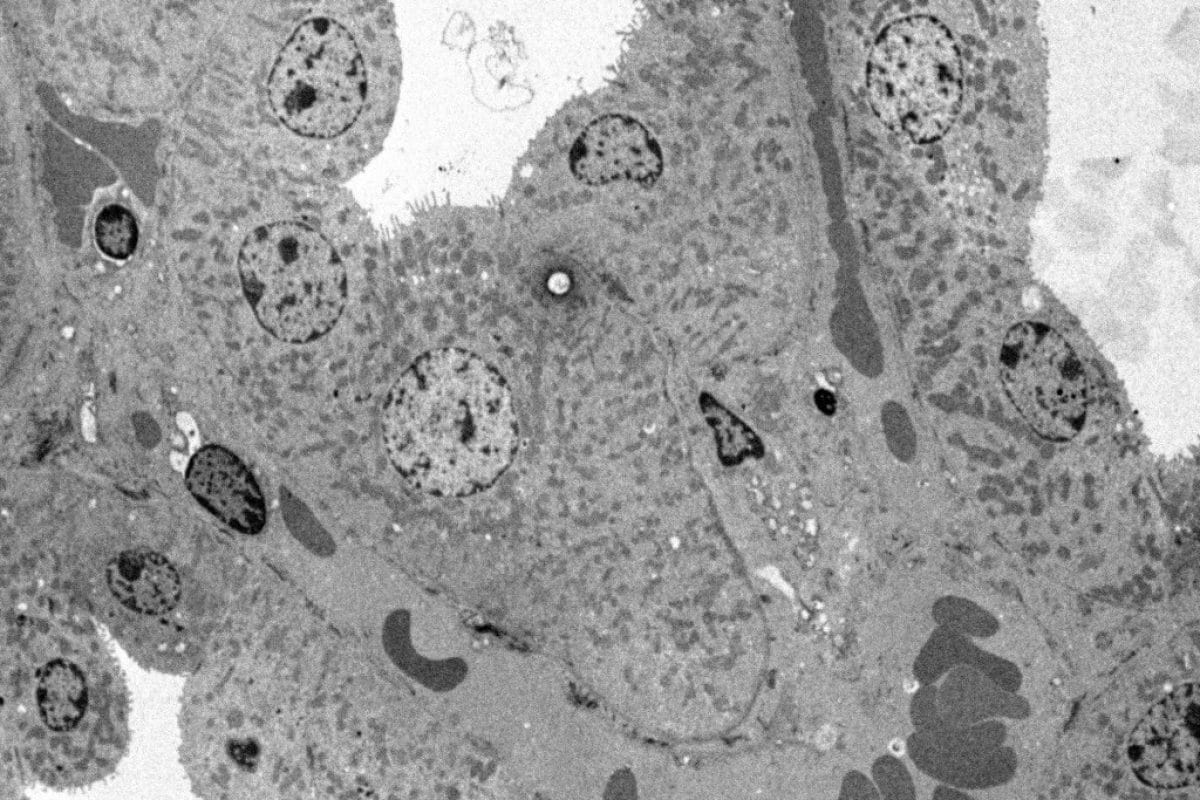
Cardiac muscle - (mouse)
Preparation of the sample using following protocol:
- classic Fixing Glutaraldehyde, Osmium, Epon Ultrafine
- Cups, Double Uranyless Contrast and Lead Citrate
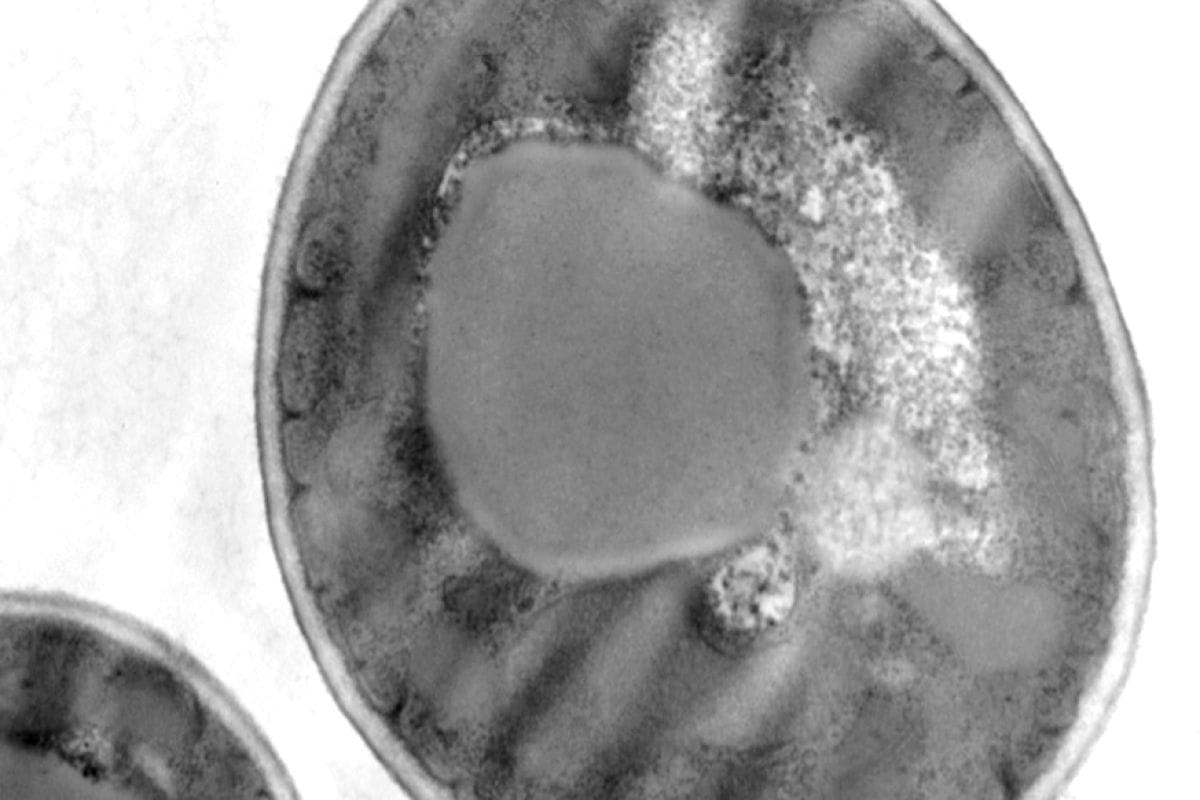
Cross sectional Bacteria (E.coli)
Sample preparation according to the following protocol: Cross sectional Bacteria
- Fixing Classic Glutaraldehyde Osmium, EPON
- Cutting ultrafine, double contrast Uranyless and lead Citrate
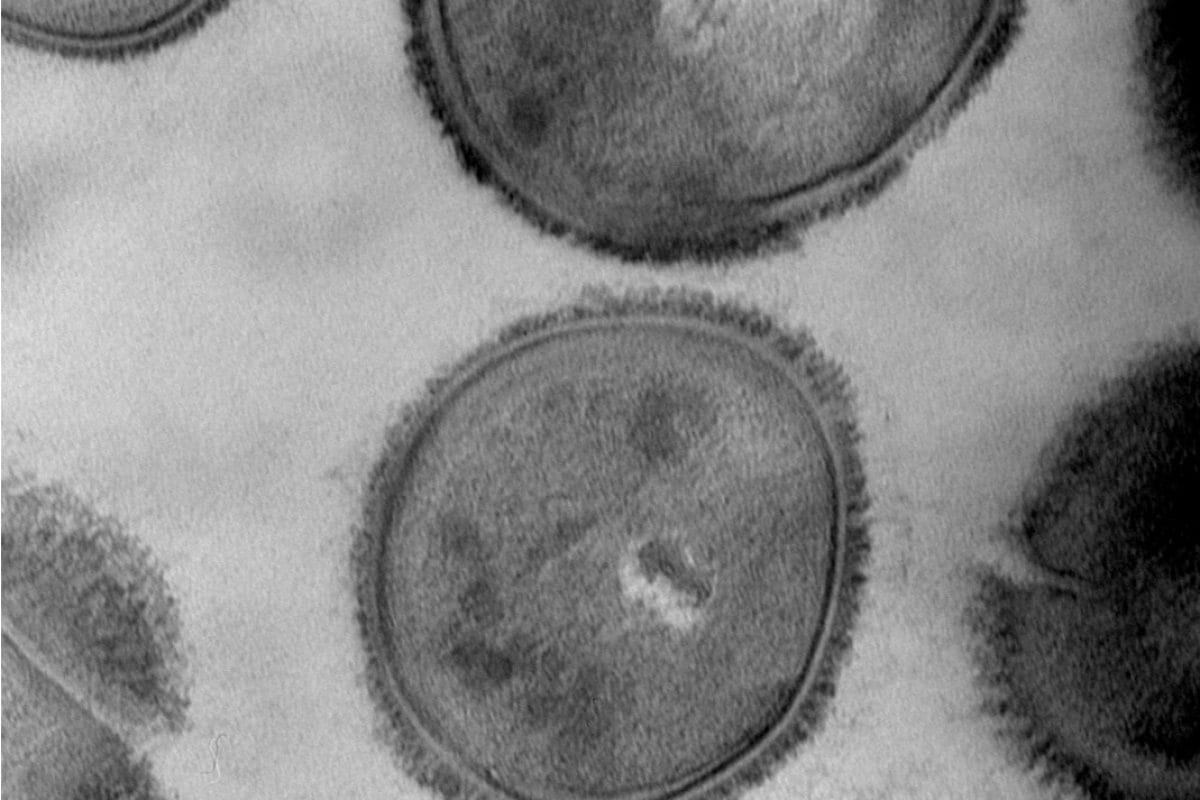
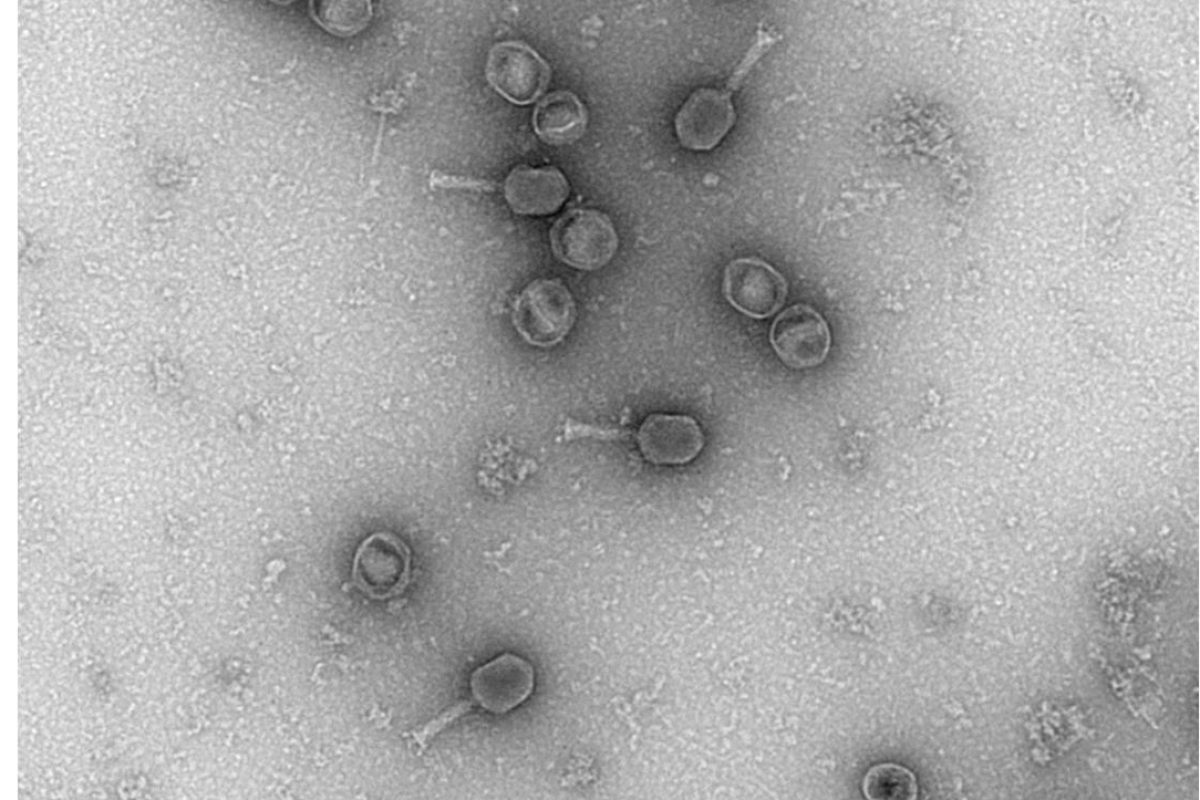
Bacteri E-coli
- Negative Staining for 2 minutes Uranyless bacteria like E-coli (adherent and invasive (ACSI) LF82) Which have Pili and Flagella .
Sacculina crustacean (Intestine)
Sample preparation according to the following protocol: Sacculina Crustace (small parasitic crustacean)
- Fixing Classic Glutaraldehyde Osmium, EPON inclusion
- Fines Cups- Contrast to the aqueous Uranyless 60°C on a Hotplate without Lead Citrat post coloring
Intestine
- Classic Glutaraldehyde Osmium, EPON inclusion
- Ultrafine cup-Contrat Uranyless and Lead Citrat
Liver Mouse and andrenal Gland
Sample preparation according to the following protocol: Liver Mouse and gherbil Sahara
- Fixing Classic Glutaraldehyde Osmium, EPON
- Cutting ultrafine, double contarst Uranyless and lead Citrat
Andrenal Gland gherbil Sahara
- Fixing Classic Glutaraldehyde Osmium, EPON
- Cutting ultrafine, double contarst Uranyless and lead Citrat
Cells in culture HeLa
Sample preparation according to the following protocol:
- Classic PFA- Glutaraldehyde, Osmium, Epon fixing.
- Ultrafine section – UranyLess contrast – Lead Citrate.
Photos taken with a Hitachi HT7700 Transmission Electron Microscope by Nacer BENMERADI (R&D – Delta Microscopies – France):
SEE ALSO
Navigation
Contact
22b route de Saint Ybars 31190
Mauressac France
+(33) 5 61 73 60 14
info@deltamicroscopies.com